Undoing
Carbon
Dioxide
逆轉
二氧化碳
排放
By Twinkle Poon
潘晴
This article may be useful as supplementary
reading for chemistry classes,
based on the DSE syllabus.
根據化學科文憑試課程綱要,
本文或可作為有用的補充讀物。
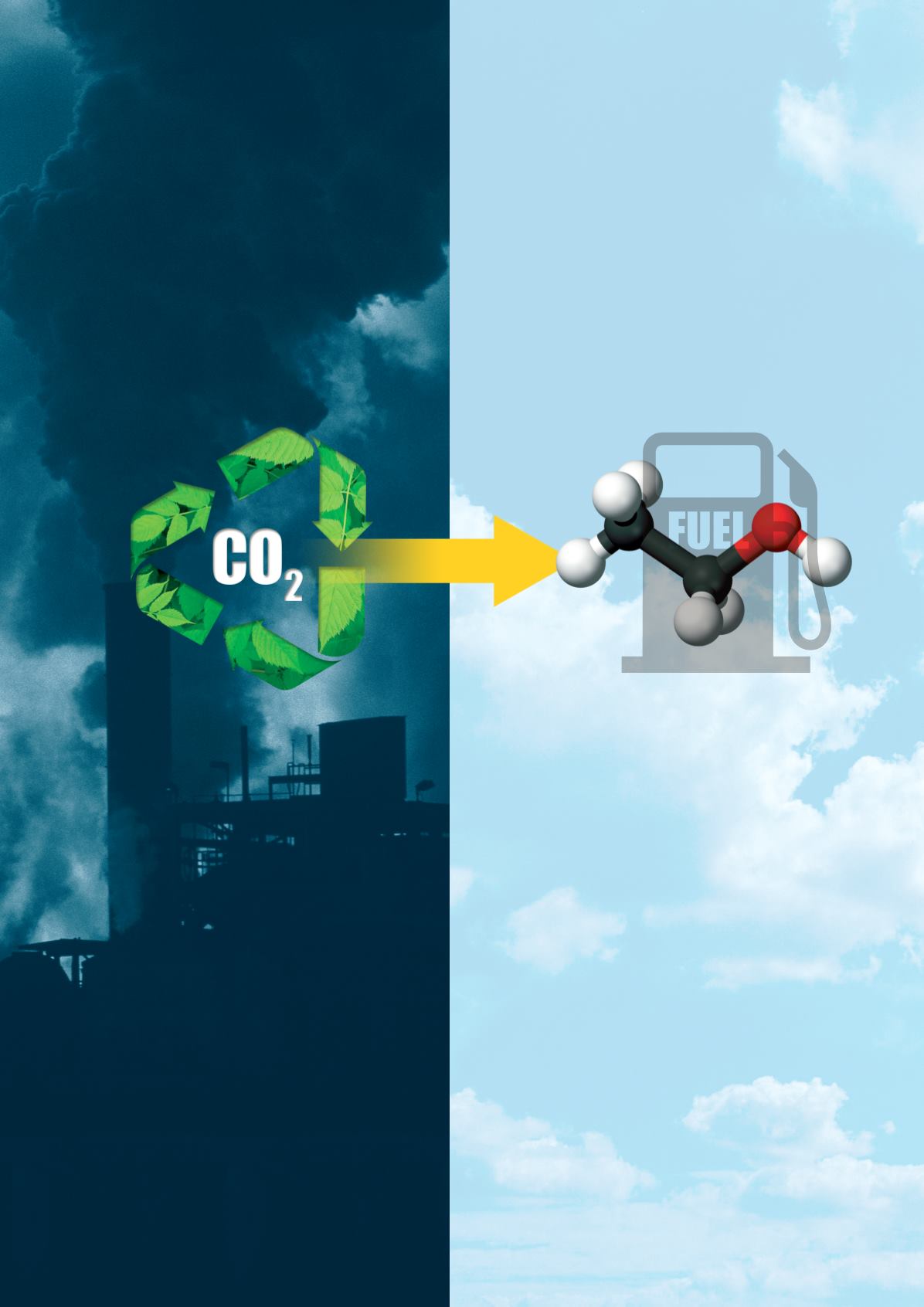
Global
warming has been an ongoing
problem for the environment due to the emission
of carbon dioxide. Global temperatures rise, as
do sea levels, causing unpredictable changes to
ecosystems. Researchers have recently discovered
a method that efficiently converts carbon dioxide
into ethanol, effectively removing it from the
atmosphere, and potentially turning it into useful
fuel.
Researchers from The Oak Ridge National
Laboratory’s Department of Energy accidentally
stumbled upon an electrochemical process that
harnesses nanotechnology and catalysts to turn
carbon dioxide into ethanol, which can be used
as a fuel.
Their original goal was to grow a graphene
based catalyst and to assist in the conversion
of carbon dioxide into methanol, which also
happens to be the first step in producing fuel.
However, they realised that the catalyst was doing
surprisingly well, so well in fact that they noted
the catalyst was doing the entire reaction on its
own, skipping anticipated steps [1]. The technique
involves using a copper catalyst designed with
copper nanoparticles on the surface of silicon,
and applying electricity to carbon dioxide in
water. Only 1.2 volts are needed to complete
the conversion to ethanol at 63% efficiency.
The reaction also works in room temperature
and can be easily switched on and off without
energy penalty, which indicates that the energy
conversion can be used as energy storage when
generating intermittent renewable energy from
wind or solar.
The scientists stated that the reaction is typically
difficult to achieve with one single catalyst [2].
The key to this reaction seems to lie with the
manipulation and design of the catalyst. While
copper itself is not an impressive catalyst, by
Ethanol


