Atomic Weight
Changed for
Elements
個元素的原子量改變
By Sunjung Lim
林宣廷
Wow
Cadmi um, you have got ten
chubbier these days!
According to the International Union of Pure
and Applied Chemistr y (IUPAC), the federation
responsible for overseeing international standards
for atomic weights since 1919, nineteen elements on
the periodic table are getting their atomic weight
adjusted. IUPAC has approved new weights for
these elements due to better measurements and
calculations of certain isotopes [1]. Scientists have
been publishing tables with atomic weights that
were determined as far back as 1899 [2].
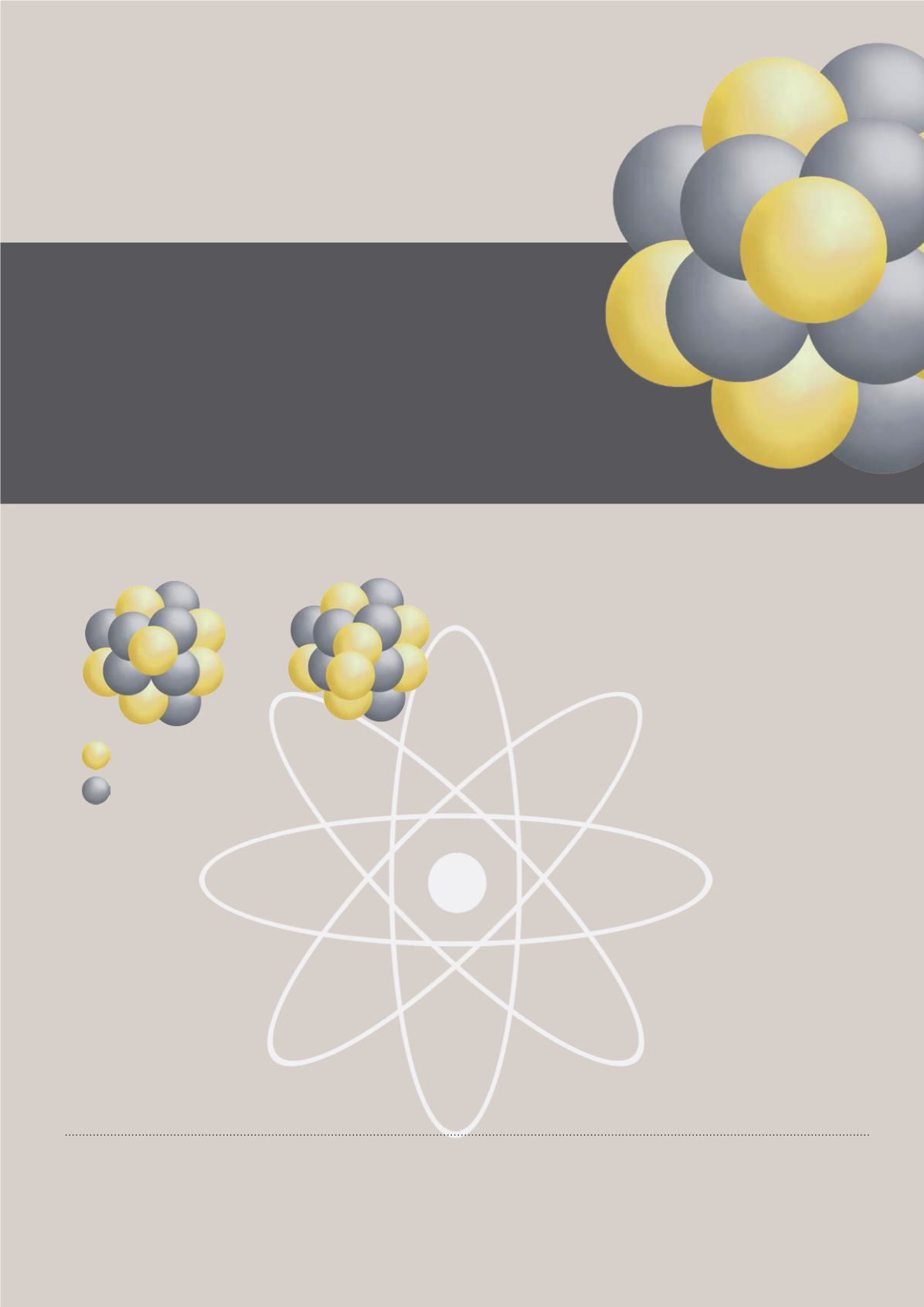
How do we calculate the atomic weight? To
use carbon as an example, a carbon atom has six
protons in its nucleus. For the atoms to be neutral
(no net charge), the number of electrons must
equal the number of protons. Thus, a carbon
atom has six electrons orbiting around its nucleus.
However, each carbon atom also has neutrons in
its nucleus, and different types of carbon atoms
exist that have different numbers of neutrons. These
different versions of element’s atoms that have the
same number of protons but different numbers of
neutrons are called isotopes. In the case of carbon,
the most abundant stable isotopes can be found as
carbon-12 and carbon-13. Chemists calculate the
carbon-12
碳
-12 99%
carbon-13
碳
-13 1%
carbon-12
碳-12
carbon-13
碳-13
neutron
中子
proton
質子
= (12 x 0.99)+(13 x 0.01)
= 11.88 + 0.13
Atomic
Weight
原子量
=
12.01
References
[1] Gannon, M. (2013, September 24). Atomic weight changed for 19 elements. Retrieved from
.
livescience.com/39912-atomic-weight-changed-for-19-elements.html
[2] Yirka, B. (2013, September 26). IUPAC votes to change standard atomic weights of 19 elements. Retrieved from
Calculation of Atomic Weight
原子量計算方式
19


